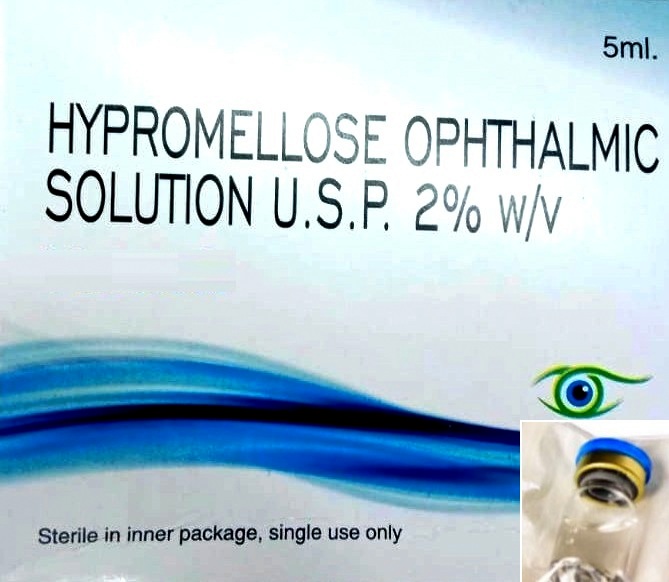
Specification Table for Viscoelastic Solution
| Specification | Details |
|---|---|
| Active Ingredient | Hydroxypropyl Methylcellulose 2% |
| Molecular Weight | High molecular weight |
| Purity Grade | Highly purified |
| Solution Characteristics | Clear, isotonic, sterile, non-inflammatory, non-pyrogenic |
| Application | Intraocular injection during anterior segment surgery |
| Key Features | Maintains anterior chamber depth, protects periocular tissues, outstanding rheological properties, completely transparent, totally non-antigenic, simple removal from anterior chamber |
| Storage Conditions | Does not require refrigeration, should not be stored above 35°C |
| Usage | Ophthalmic surgical procedures, particularly anterior segment surgery |
| Packaging | Sterile, single-use packaging |
| Transparency | Completely transparent for clear visibility |